Harrit Clarifies Proof of NanoThermite in the Red/Gray Chips
Bob Greenyer streamed a [1]live YouTube video [1] on October 5, 2025 attempting to debunk the 2009 peer-reviewed paper published in the Bentham Open Chemical Physics Journal, Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center Catastrophe [2]. The following is the response by lead author Niels Harrit, dated October 19, 2025. Harrit’s response was composed originally in three PDF documents [3].
Copenhagen, October 19th, 2025
Reply to Bob Greenyer video: Ending the Nano-Thermite 9/11 Hypothesis for the World Trade Center Catastrophe [4].
SUMMARY
1. Yes, there are anti-corrosion paints which contain both iron oxide and aluminum.
2. No, there is no information implying that they were used in the WTC twin towers.
3. No, said protective paint is definitely NOT the material described in the 2009 [2] nanothermite publication [2]:
 [2]
[2]BACKGROUND
As outlined in the Bentham paper, the red-grey chips showed properties distinctly different from random, ordinary paint, in particular towards dissolution in organic solvents. Beyond that, we did not make a big thing out of the “ordinary paint” option. After all, ordinary primary paint, rigorously tested for fire resistance, should not react violently at elevated temperature and form elemental iron, right?
After publication of the paper in 2009, we obtained an original sample of WTC-primer paint from Clarkson’s World Trade Center Memorial Sculpture in Potsdam, NY (https://northcountrynow.com/stories/clarkson-plans-911-tribute,321847 [5]). It was found to be a material distinctly different from the red/grey chips of the Bentham paper as shown by optical band electron spectroscopy.
The Potsdam sample was also subjected to thermogravimetric analysis in which the mass of the sample is measured over time as the temperature rises as a ramp. Thus, you can detect any chemical reaction or phase change. It was heated to 800 °C and nothing happened beyond a gradual charring as expected for ordinary paint.
At the time, we did not consider these findings sufficiently comprehensive to carry a full paper. That might have been short-sighted.
BASIC PRINCIPLES FOR ANTI-CORROSION MEASURES ON IRON
Very simplified, there are two complementary principles for protecting iron against corrosion, either
- Reductive, like chromium-plating and galvanization. The coating regenerates elemental iron, but – for the same reason – is less resistant to a corrosive environment than the iron itself.
- Oxidative, like red lead paint on ships and the primer paint used in WTC – to be seen. It seals off the iron and is less resistant to oxidation (because it itself is oxidized). It can be used in challenging environments like seawater or high temperatures, or if longevity is a demand.
It is straightforward to try to get the best of two worlds by making a paint which contains both a reductive agent (aluminum) and an oxidative (iron oxide). As they are antagonists, there must be limits as to the concentrations of these components, the flammability of the matrix (binder) in which they are contained, and to applications for this protective paint.
“IT IS JUST ORDINARY PRIMER PAINT”
From the start of the publication of the nano-thermite paper, dismissal of the findings as being ”just ordinary primer paint” from the WTC steel structure was abundant.
It was the general consensus that – in accordance with NIST – there was only one type of primer paint – manufactured by Tnemec – used on the columns of WTC.
As shown already in 2009 (essay attached), this primer paint had a composition distinctly different from the red/grey chips in the WTC dust.
Later it appeared that a different primer paint was used on the floor truss system. This primer (Leclede) contained pigments Iron Oxide (55%), Aluminum Silicate 41%, and Strontium Chromate (4%).1 [6]
Of course, the primer paints used in WTC were subjected to rigorous fire testing up to ca 800ºC.
Thus, there is no basis for anticipating that the common anti-corrosion paint described by Mr. Greenyer should have been applied in WTC or found its way through the collapse inferno from other sources.
Nor does it matter, as we shall see.
THE MANHATTAN BRIDGE
It wasn’t until May 2011 that the aluminum/iron oxide variant was introduced.
It happened in this BBC interview: [7]
Go to 1.32.00. Two scientists from Carnegie-Mellon(!) University skim the paper and deem it not even worth reading, least of all publishing.
At 1.36.09. One of them, Prof. Pistorius, claims that the red-gray chips are just a common form of protective paint containing iron oxide and aluminum.
At 1.42.52. This type of paint is used on the Manhattan Bridge (“Did you know that?” No, I didn’t.).
The anti-corrosion paints used on the Manhattan Bridge are enumerated here (updated website):
https://wassercoatings.com/wp-content/uploads/2020/12/Manhattan-Bridge-New.pdf [8]
As can be seen, it is only the cables that are protected by an aluminum-containing variety, called Mio-Aluminum.
Product information: https://www.dot.ny.gov/divisions/engineering/technical-services/technicalservices-repository/details/coroprime.pdf
Safety sheet: https://www.dot.ny.gov/divisions/engineering/technical-services/technicalservices-repository/details/mcmal_%20msds.pdf
As seen from the product information, it is recommended for ”normal” conditions. At the time, we could not obtain any information on the properties of the hardened material, in particular its thermal stability up to and beyond 500°C.
As the last attempt, we ended up submitting a FOIA to the Department of Transportation, New York City. It was rejected (see attached).
So we don’t know if this stuff will react in a fire. It is hard to believe. There is no nanotechnology here. The components are just mixed macroscopically. In a standard iron oxide/aluminum thermite mixture, the ratio is 3/1 (w/w). In MioAluminum, it is 1/1. So it is relatively reductive.
It may also be noted that MC-MioAluminum contains 0-1 % quartz/silica as the only component with silicon according to the safety sheet (of the wet paint). This is as close to ”nothing” as you can get. In contrast, the red/grey chips of our findings contain silicon as an essential component.
On the basis of this alone, the MC-MioAluminum paint variety on the Manhattan Bridge cables cannot be the same material as found in the red/grey chips.
“ACTIVE”
Mr. Greenyer.
Did you ever READ the title of the paper you dismissed?
”Active” means ”Reactive”.
The chips are reacting, forming elemental iron and aluminum oxide in the process.
This is the signature reaction of the (most common) thermite reaction. That is why the red/grey chips are categorized as active thermitic material.
In your introduction (20.28), you quote from the abstract: ”Numerous iron-rich spheres are clearly observed in the residue after ignition of the red/grey-chips.”
Whereafter, you announce that “this last comment will not be the focus of this particular talk. This talk will just be dealing with removing the nano-thermite idea entirely using historical understanding and the facts.”
Indeed, in your 2-hour podcast, you spend barely 2 minutes (43.20 – 45.13) being defocused on the reactivity of the red-grey chips.
During these 1 min 45 sec you successfully ignore, misrepresent, misunderstand, and misinterpret the key findings of the paper. Add to this a little dash of nonsense to make it all the more indigestible.
FROM THE TOP:
Fig. 19 shows Differential Scanning Calorimeter (DSC) traces from each of the four samples investigated. You skip this decisive observation entirely, although it directly illustrates the highly energetic reaction of the chips.
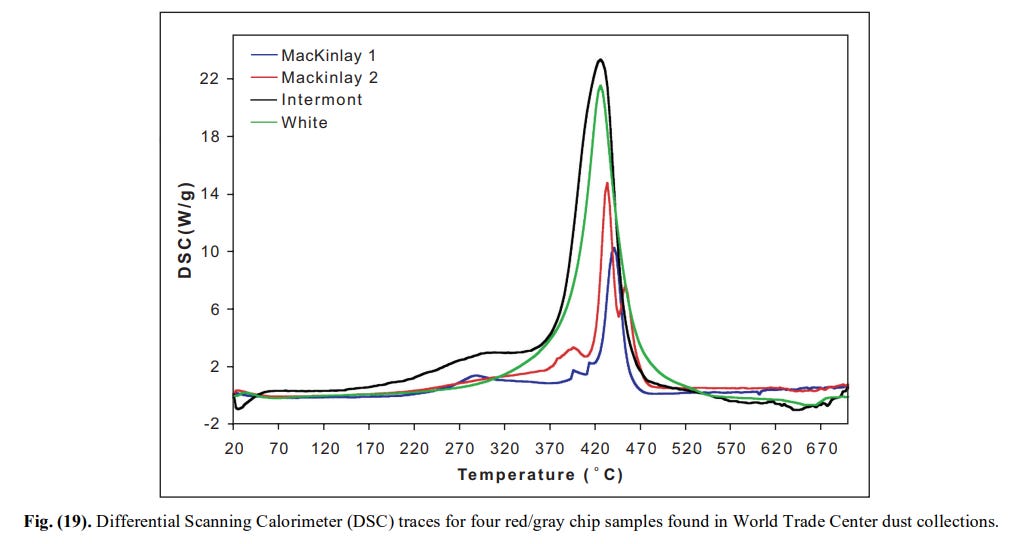 [9]
[9]Fig. 20 shows the residues from the DSC. You falsely claim (43.35), that they are obtained from exposure to the microtorch. Read the caption.
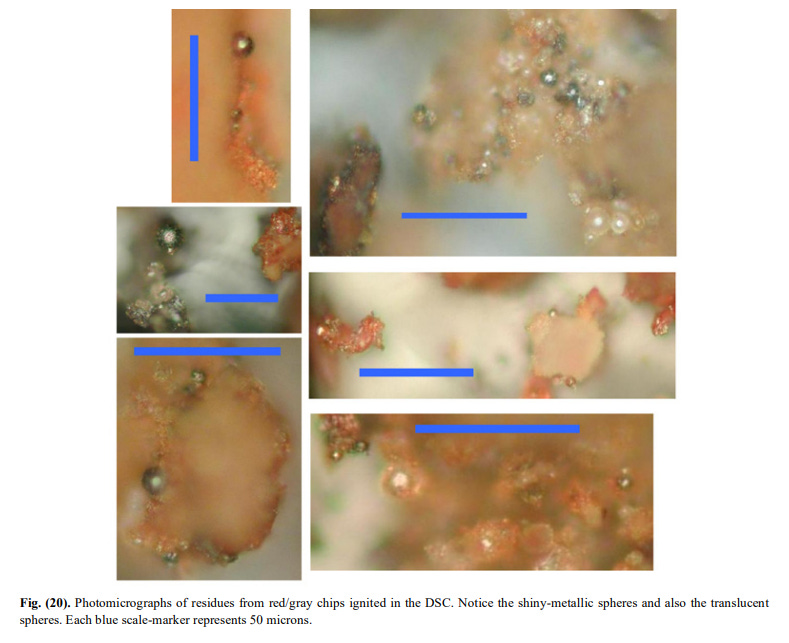 [10]
[10]What you see in Fig. 20 are completely spherical metallic spheres (some silicates too). The XEDS of one of these are seen in Fig.21.
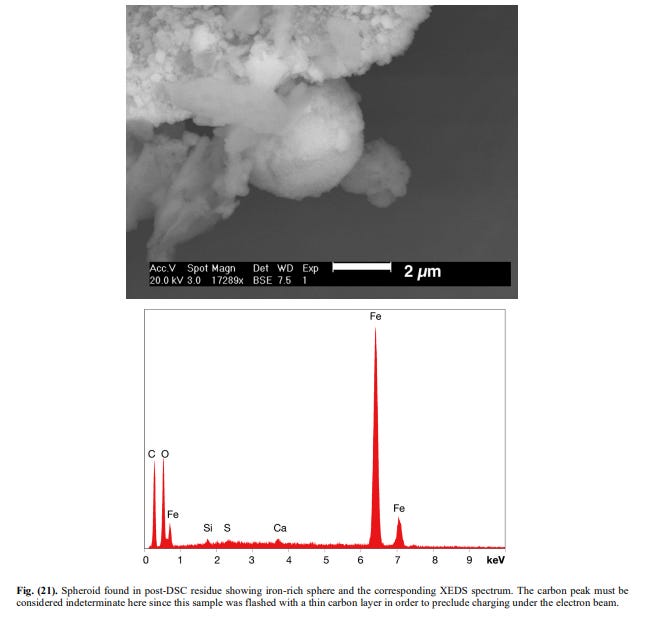 [11]
[11]This is pure iron, plain and simple.
Other spheres coming out of the DSC showed other elements too and must be termed just ” iron-rich” (Fig 25).
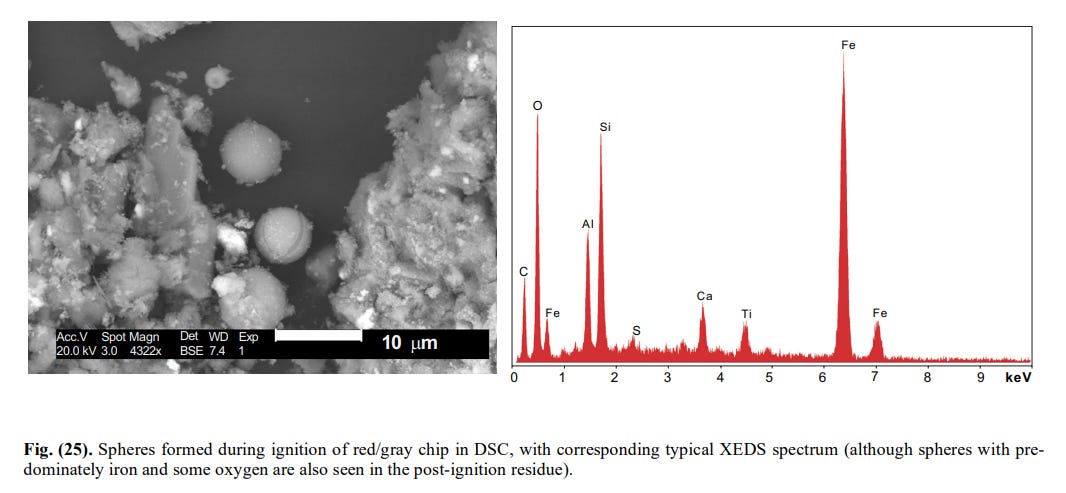 [12]
[12]The same diversity was observed among the iron (rich) spheres coming out of the WTC dust itself. Discussion of this heterogeneity is irrelevant here. Suffice to notice that the XEDS of a sphere from the DSC (Fig. 25) is identical to the XEDS of a sphere from the WTC dust (Fig. 27).
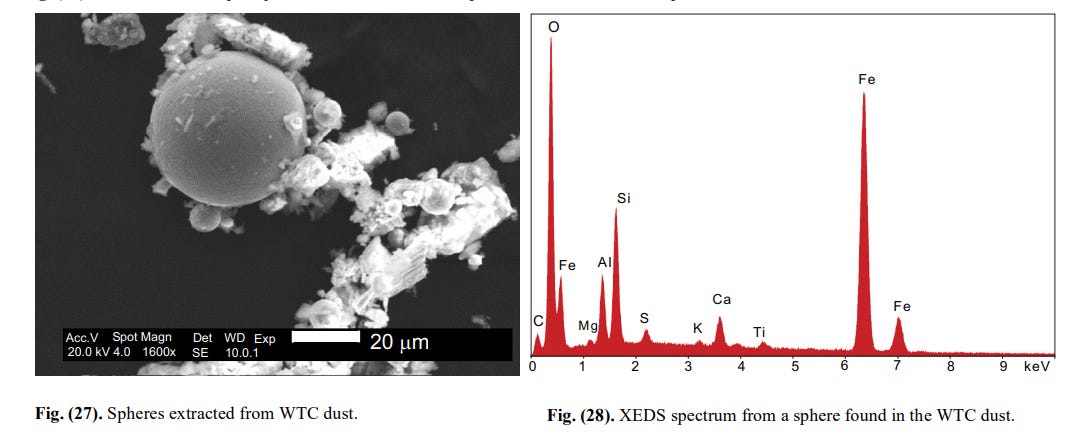 [13]
[13]Quote Bob G. at 43.37: “When you melt anything that has metal in it, you gotta get little spheres.”
Eh…Melt? Metal? What metal melts?
You appear to understand the mechanism of surface tension and why the water droplets in the morning mist are completely spherical.
So to form spheres spontaneously, the material must be in the liquid state. But since there is no other metal to melt than iron,2 [14] your own – correct – observation implies that the iron must have been beyond its melting point at 1538 °C for some time. It doesn’t matter for how long. In the nanoworld, milliseconds can be eternity.
Considering that the surface/volume ratio increases dramatically when things get into sub-micron dimensions,3 [14] the heat dissipation from the sample in the DSC must be enormous compared to the weight. We must therefore conclude that the energy released from the chip when ignited around 430 °C must also be enormous for the products to go to, say, 1600 °C. The heat capacity of the DSC is far too big to register that.
Thus, the simple qualitative observation of the particles being round – for everyone to make – is far more powerful and decisive than any number coming out of the DSC.
Actually, it tells ALL.
Fig. 22. Applying a small torch to a minute red chip.
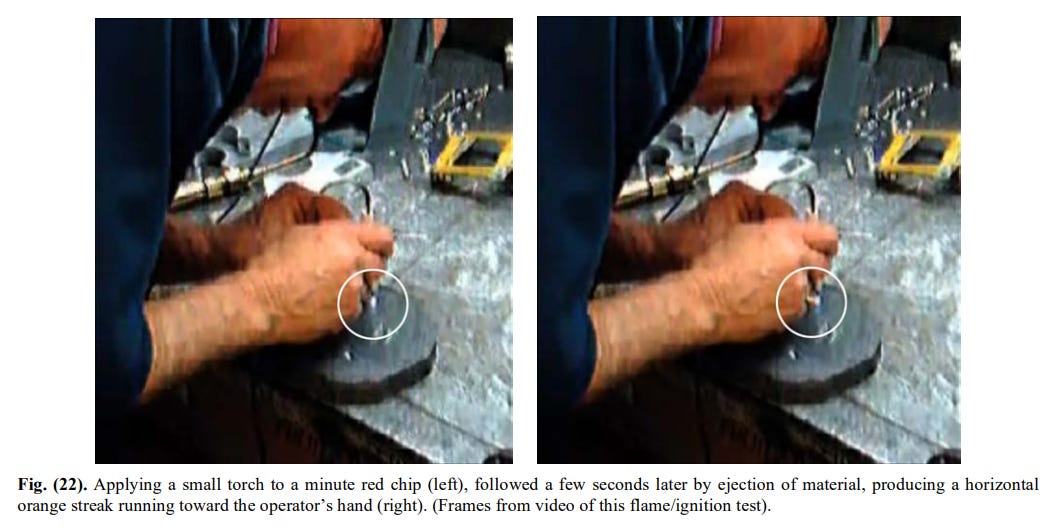 [15]
[15]You ignore the fact that the thing MOVES when it reacts. That is, there must be a thrust. It can only come from the formation of a gas.
As to the resulting almost-sphere (Fig. 23), you comment (43.47): “Here is something that looks a little bit spherical. To me it looks just like a little bit of molten metal. It is not really a sphere”.
Yeah. It just barely made it to 1600 °C. The temperature in a microtorch can never get close to that, and the gas pressure is relatively high. So the iron being formed is apparently being cooled faster than in the DSC. Please notice that the XEDS of this sample (Fig. 26) is identical to that of a sphere from the WTC dust (Fig. 27) – disregarding the gypsum and some white paint (TiO2).
ALUMINUM OXIDE
As to the formation of white aluminum oxide you may observe it [in the David Chandler videos] here [16],
here, [17]
here, [18]
and here [19].
SO….
Mr. Greenyer.
If you think that your “standard anti-corrosive paint” can react like that, you may take a seat on the bank of the East River, NYC, and wait for some Tesla to catch fire on the Manhattan Bridge. The chain reaction that should follow in the cables of the bridge is bound to be pretty spectacular.
Rather, do what any decent scientist would do: Take your stuff to a well-equipped lab and repeat the experiments described above. Show that your standard anti-corrosive paint can reproduce our results. Write up your findings into a regular manuscript and submit it to an open, peer-reviewed journal for everyone to evaluate. Then take your findings to a plenum of unbiased scientists and let us have a discussion.
In that case: GREAT!
If you can also document that this standard anti-corrosive paint was used in WTC, it starts getting really interesting.
In anticipation,
Niels Harrit, Ph.D.
Associate Professor (ret.)
Nano-science Center
Dept. Chemistry, University of Copenhagen
Footnotes
1. Fire Resistance Tests of Floor Truss Systems. Specifications for Leclede primer paint is found on p. 157. https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=101042 [20]
2. One way or another any elemental aluminum is gone, either by reacting with the iron oxide or reacting with atmospheric oxygen. Actually, molten aluminum can burn!
3. This is the key to understanding nanotechnology. It is all about the interface.